This blog has been written to support a recent session I delivered for ACCS trainees on ‘Syncope’. This is not exhaustive but aims to explore some of the more interesting snippets of information I found on various FOAM resources.
What is syncope?
Syncope is a loss of consciousness due to temporary cerebral hypoperfusion. Therefore syncope is not the same as transient loss of consciousness (TLoC) which is a much broader term for any blackout.
A key feature of syncope is that it is transient; a short period of collapse with a short recovery. 30% of patients with syncope will have more than one episode.
Syncope by itself makes up 3-5% of Emergency Department presentations.
In 50% of these cases we don’t find a cause. Investigating a patient costs about £2000.
In those patients where we find a cause neurocardiogenic syncope or vasovagal syncope is the most common form of syncope. This is caused by an initial increase in sympathetic outflow followed by a rebound reduction in sympathetic activity leaving unopposed parasympathetic activity. Vasovagal syncope makes up 35% of causes.
Pathophysiology of vasovagal syncope from RCEMLearning, 2018
Key Point: Remember the 3Ps of vasovagal syncope: Prodrome, Posture and Provoked. Don’t try and shoehorn a diagnosis!
Orthostatic syncope is due to an orthostatic drop >20mmHg systolic or >10mmHg diastolic. This could be due to a reduction in circulating volume (haemorrhage or dehydration) or vasodilatation due to medications or autonomic dysfunction (such as Parkinson’s). Orthostatic syncope makes up 10% of cases.
Cardiac syncope makes up 10-30% of cases. This includes arrhythmias, heart failure and structural and valve problems.
Neurological/psychiatric syncope is the rarest cause at 5% of cases. Neurological causes include basilar artery migraine, vestibular dysfunction and vertebrobasilar ischaemia. Psychiatric syncope is a recognised syndrome found in patients with anxiety, depression and conversion disorder that resolve with treatment of the psychiatric disorder.
Classification of syncope from RCEMLearning, 2018
Whilst cardiac syncope is not the most common causes of syncope it is associated with the highest mortality.
Mortality of the various aetiologies of syncope from Salim Rezaie, 2018
Key point: Syncope is common, most of the time we don’t find a cause but there are some very serious causes with high mortality
Because of how common syncope is and the potentially severity there are a few risk stratification scores designed to help us with the assessment of patients presenting with syncope.
One such score is the San Francisco Syncope Rule which tries to identify high risk patients at risk of a serious outcome (death, MI, arrhythmia, PE, stroke, subarachnoid haemorrhage, significant haemorrhage or any other condition causing a return ED visit or hospitalisation for a related event) in the next 30 days. It uses the mnemonic ‘CHESS’:
C ongestive heart failure
H aematocrit <30%
E CG abnormal (changed or any non-sinus rhythm)
S hortness of breath
S ystolic BP <90mmHg at triage
If ‘Yes’ is answered for any of these then the patient can’t be considered ‘Low Risk’.
If you think about these are all very sensible criteria covering pump failure, potential bleeding, arrhythmia, PE and hypotension.
So far so good but what about the evidence? MdCalc tells us that The San Francisco Syncope Rule has 96% sensitivity (not surprising given how broad the criteria are) but only 62% specificity so we’d still be scoring about a third of patients without a serious cause as a high risk patient. If a patient is deemed low risk the NPV is 99.2% but PPV for those deemed high risk is only 24.8%. According to MdCalc then it will pick up most people with a serious cause (96%) but there are a lot of false positives (75.2%) and whilst it delivers few false negatives (0.8%) it will also fail to rule out about a third of people without a serious cause. Plus, whilst it is good that it gets us thinking about certain ‘never miss’ diagnoses like PE we already have the Well’s Score for that anyway for more constructive risk stratification.
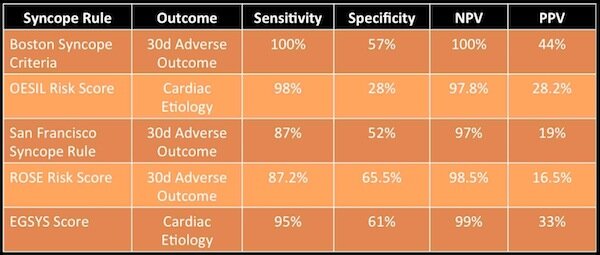
Academic Life in Emergency Medicine (ALIEM) from Australia has different data but shows similar issues with the San Francisco Syncope Rule and other scoring systems:
Summary of syncope risk stratification scores from Salim Rezaie, 2018
Key point: Syncope risk scores are useful but not enough. Clinical assessment should always override
So we can’t escape a good history and examination. Ask about before, during and after with all collapses.
Before – what were they doing? Were they lying down, standing up, exerting themselves, on the toilet, coughing or swallowing? Were they stood up in a hot, crowded place or had they just eaten? Was there a prodrome? What medications are they on? Have they been changed? Is there a family history of sudden death including unexplained drownings or accidents? Any chest pain, headache, abdominal pain or shortness of breath? Any recent illness? Anything like this before?
During – do they remember what happened? Get a collateral history of what they were doing while collapsed. How did they look? What was the duration?
After – How quick was the recovery? Are they back to normal? Any deficits? Any injuries? Did they bite their tongue or were they incontinent? Any nausea or vomiting? Any confusion?
A thorough cardiovascular examination is essential checking ECG, JVP and murmurs.
Specific findings in the cardiovascular examination from RCEMLearning, 2018
RCEM advise that tachycardia and hypotension point toward volume depletion and so should make us concerned. Lying and standing BP should be sought.
Neurological examination isn’t so useful; abnormalities found may not point to the pathology and a normal examination does not rule out a neurological cause.
Whilst tongue biting suggests seizure it is not sensitive and its absent does not exclude seizure.
Elderly patients are likely to have two or more reasons for their collapse. Finding one cause does not preclude from their being others.
Increasing frequency of collapse suggests cardiac causes of syncope. If the patient is known to have cardiac pathology this is an ominous sign.
Simple syncope during exercise is rare. The presence of exertional syncope is strongly suggestive of either an arrhythmia or a structural cardiac abnormality.
Differential diagnoses of TLoC from RCEMLearning, 2018
Key Point: Your patient with syncope is a ‘WOBBLER’
WOBBLER’ is a great mnemonic for remembering key ECG findings to look for in any case of syncope. If I hear that the ECG is from a patient with syncope I usually write ‘WOBBLER’ and tick off each one as I’ve checked for them. It also goes through the order of the P, QRS and T waves.
ECGs and diagrams from Life in the Fast Lane
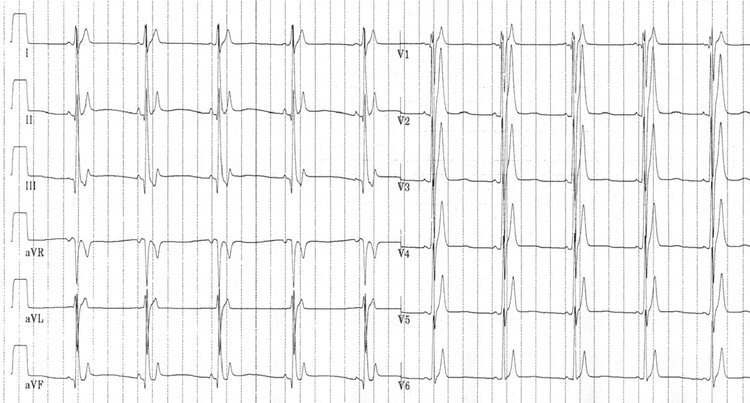
Wolff-Parkinson-White
Wolff-Parkinson-White is a pre-excitation syndrome caused an accessory pathway called the Bundle of Kent which bypasses the AV node. The classic features are of a short PR interval and the delta wave. There are two types of WPW. In Type A WPW the accessory pathway is left-sided and produces a positive delta wave:
Type A Wolff-Parkinson-White
In Type B WPW the accessory pathway is right sided and produces a negative delta wave:
Type B Wolff-Parkinson-White, the negative delta wave is in III and aVF
Because of this bypassing of the AV node the patient is at risk of going into a tachyarrhythmia. There is a small risk of sudden death. Electrophysiology studies confirm the presence of the accessory pathway which is then ablated.
For more on Wolff-Parkinson-White check out our blog here.
Obstruction of AV Node
Mobitz II and Third Degree Heart Block are both linked to sudden cardiac death.
Mobitz II
Unlike Mobitz I which is usually a functional suppression of AV conduction through drugs or reversible ischaemia Mobitz II is more likely due to structural damage to the conducting system through infarction, fibrosis or infection. Mobitz I is progressive fatigue of the AV nodal cells but Mobitz II is an ‘all or nothing’ phenomenon where the Purkinje cells suddenly and unexpectedly fail to conduct a supraventricular response.
Third Degree/Complete Heart Block
There is complete absence of AV conduction. The patient relies on junctional or ventricular escape rhythm. The patient is at risk of ventricular standstill causing syncope if self-limiting or death if prolonged.
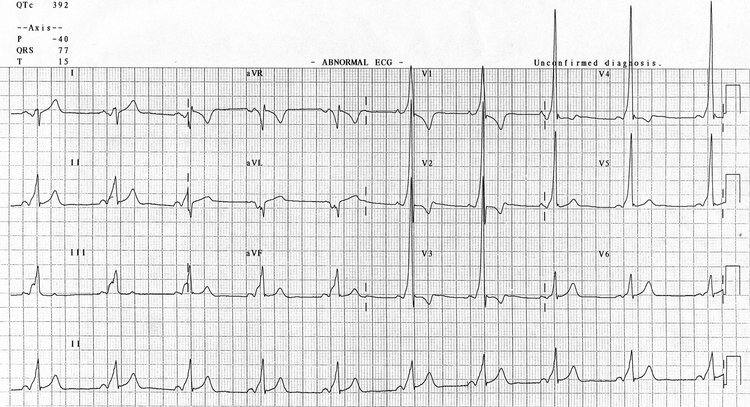
Brugada
Brugada syndrome is due to a sodium channel gene mutation. There is a familial link and autosomal dominant inheritance has been shown. Type 1 Brugada, the only definitive ECG change which is potentially diagnostic, is this coved ST segment elevation in V1-3 followed by the negative T wave. This is Brugada sign. ECG changes can be unmasked by fever, ischaemia, drugs such as sodium channel blockers, calcium channel blockers, beta blockers, alcohol and cocaine, hypokalaemia and hypothermia.
Type 2 Brugada has a saddleback ST elevation >2mm. Type 3 can look like either 1 or 2 but the elevation is <2mm. 2 and 3 are not diagnostic but warrant further investigation.
The only proven therapy is an implantable cardioverter-defibrillator. Untreated Brugada is estimated to have a mortality of 10% every year. Risk stratification and management of asymptomatic patients is controversial.
Bifasicular block
The conducting system is divided into a right bundle branch and a left bundle branch which is then further divided into an anterior and a posterior fascicle.
In bifascicular block the right bundle and one of the left anterior or posterior bundles are blocked. This creates a RBBB with either a left (LAFB) or right axis deviation (LPFB). This shows there is a serious problem with conduction although progression to complete block seems to be rare.
Bifascicular block showing RBBB and LAFB
Causes of bifascicular block include ischaemic heart disease (most common), hypertension and aortic stenosis.
Trifascicular block refers to blocking of the right bundle and both left fascicles. This can be incomplete or complete. Incomplete refers to bifascicular block with either a 1st or 2nd degree heart block:
Complete trifascicular block looks like bifascicular block with 3rd degree AV block:
Again there is a risk of complete heart block. Causes for trifascicular block are similar to bifascicular. Hyperkalaemia also causes it – this resolved with treatment – as does digoxin toxicity.
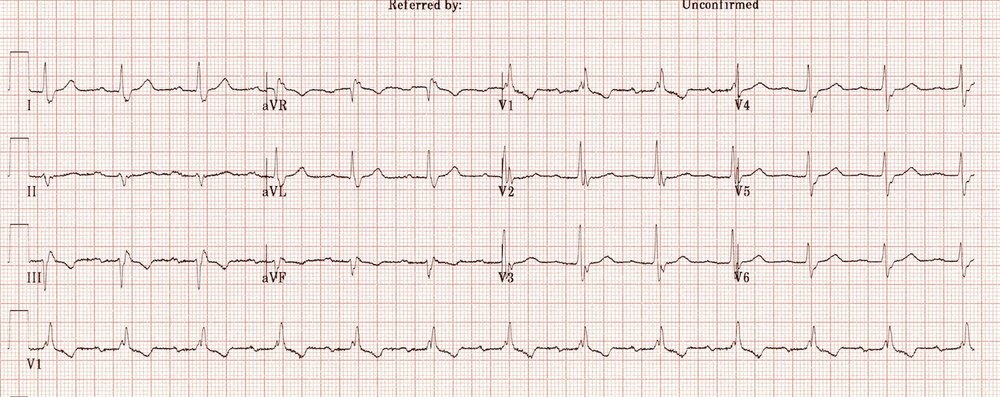
Left Ventricular Hypertrophy
On this ECG you can see the markedly increased LV voltages: huge precordial R and S waves that overlap with the adjacent leads. This is classic for LVH.
Hypertension is the most common cause of left ventricular hypertrophy. Other causes include aortic stenosis and regurgitation and structural problems such as coarctation of the aorta and hypertrophic cardiomyopathy. It’s worth remembering that voltage criteria alone is not diagnostic and ECG changes are insensitive for left ventricular hypertrophy.
Epsilon wave
The epsilon wave is a small positive ‘blip’ buried at the end of the QRS complex. It is characteristic of arrhythmogenic right ventricular dysplasia (ARVD).
ARVD is an inherited myocardial disease associated with paroxysmal ventricular arrhythmias and sudden cardiac death. The right ventricular myocardium is replaced by fibro-fatty material. After HCM it is the second most common cause of sudden cardiac death in young people (20% < 35 years). The epsilon wave is seen in 30% of patients with ARVD. You may also see anterior T wave inversion. Echocardiography is the first-line investigation but MRI is often the imaging modality of choice.
Repolarisation
Short QT syndrome is a recently discovered (2000) arrhythmogenic disease associated with AF, VF, syncope and sudden cardiac death. It is an inherited channelopathy. It is a possible cause of sudden infant death.
Short QT
There are no diagnostic criteria for short QT syndrome but the ECG features are a short QT interval, short ST segments and peaked T waves especially in the precordial leads:
Long QT syndrome is a congenital disorder causing a prolongation of the QT interval with a propensity to ventricular tachyarrhythmias. Hypomagnesaemia, hypocalcaemia and hypokalaemia can cause long QT. Many drugs can also be responsible such as amiodarone, many antibiotics, TCAs, ondansetron, SSRIs and haloperidol.
Long QT
Key Point: Who’s gonna drive them home?
For a nice PDF table regarding DVLA regulations and certain conditions have a look at here
For simple vasovagal syncope there are no driving restrictions for either Group 1 or Group 2 drivers. The DVLA does not need to be notified for either group.
In cases of unexplained syncope but the likelihood is vasovagal syncope again there are no restrictions for Group 1 drivers. Group 2 drivers can’t drive for 3 months.
In unexplained loss of consciousness with high risk factors (or more than one episode in 6 months) Group 1 drivers can’t drive for 6 months if no cause is identified (can drive after 4 weeks if a cause is found). For Group 2 drivers they can drive 3 months after if a cause is found, 12 months if no cause is found.
In cough syncope Group 1 drivers must stop for 6 months for a single episode and 12 months for multiple. Group 2 drivers cannot drive for 5 years from the last attack.
In arrhythmia Group 1 drivers must cease if it has caused or is likely to cause incapacity. If the cause is found and controlled they may drive after 4 weeks. The DVLA doesn’t need to be told unless there are distracting/distracting symptoms. Group 2 drivers are disqualified.
Syncope is a common and sometimes challenging presentation. Hopefully this review has shown you a few tips and key snippets to help you when you assess your next patient with syncope. Syncope? Cope.
- Jamie
References:
Marjorie Lazoff, M., Marjorie Lazoff, M., Davies, A., Cadogan, D. and Marjorie Lazoff, M. (2018). LITFL Life in the FastLane. [online] Life in the Fast Lane • LITFL • Medical Blog. Available at: https://lifeinthefastlane.com/ [Accessed 12 Sep. 2018].
Mdcalc.com. (2018). San Francisco Syncope Rule - MDCalc. [online] Available at: https://www.mdcalc.com/san-francisco-syncope-rule#evidence [Accessed 12 Sep. 2018].
Rcem.ac.uk. (2018). [online] Available at: https://www.rcem.ac.uk/docs/College%20Guidelines/5z33.%20RCEM%20summary%20of%20DVLA%20fitness%20to%20drive%20medical%20standards.pdf [Accessed 12 Sep. 2018].
RCEMLearning. (2018). Syncope - RCEMLearning. [online] Available at: https://www.rcemlearning.co.uk/references/syncope/ [Accessed 12 Sep. 2018].
Salim Rezaie, M. (2018). Management of Syncope. [online] ALiEM. Available at: https://www.aliem.com/2013/04/management-of-syncope-aka-done-fell-out/ [Accessed 12 Sep. 2018].